is salt a heterogeneous mixture
Homogeneous mixture Heterogeneous mixture. A difference between the two is that concentration is same everywhere in.

Let S Do An Experiment Homogeneous And Heterogeneous Mixtures Heterogeneous Mixture 5th Grade Science Physical Changes Experiments
The question arises whether the saltwater is homogeneous or heterogeneous.

. Chapter 171 Questions 1. Salt water solution is a homogeneous mixture for example but salt mixed with sand is a heterogeneous mixture. Thank you for asking the question.
Heterogeneous vs Homogeneous Mixtures Sometimes its obvious a mixture is heterogeneous like a stream bed of different sizes shapes and colors of rocks. Chocolate chip cookies and soda are examples of heterogeneous mixture. Answer 1 of 5.
Salt iodisation is not a chemical process and iodised salt is not a chemical compound. An example is saltwater. Is table salt an element a compound a heterogeneous mixture or a homogeneous mixture.
A mixture can be homogeneous or heterogeneous. A mixture is an example of water. Salt and pepper form a heterogeneous mixture.
Class 9 Is matter around us pure Tagged With. It is a homogeneous mixture and a heterogeneous one. Air is made up of several gases in different amounts.
If the salt in your question is sodium chloride it is an ionic compound made up of sodium ions and chloride ions. If the salt in your question is the substance in the salt shaker on your table i. As other responders have pointed out weve got to be careful about language.
B Because the composition of the solution is uniform throughout it is a homogeneous mixture. Heterogeneous mixtures consist of different substances. With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl.
For example a mixture of common salt and sand is heterogeneous whereas that of salt and water is homogeneous. A Tea is a solution of compounds in water so it is not chemically pure. Saltwater includes dissolved salt so its a homogeneous mixture together we cant different salt from that directly.
Types of Heterogeneous Mixtures. Water is a homogeneous mixture of nitrogen oxygen and smaller amounts of other compounds in the gaseous materials. Water is a homogeneous mixture of nitrogen oxygen and smaller amounts of other compounds in the gaseous materials.
But all of the constituent particles of air are gases so it is a homogeneous mixture. Is milk a homogeneous Why. But when you add sand which is insoluble you will have a mixture of two different phases a solid and a liquid therefore this is a heterogeneous mixture.
But it is likewise called a heterogeneous mixture due to the fact that of the presence of impurities and insoluble materials like sands shells made up of calcium carbonate and also microbes in it. Salt gets completely dissolved in water and salt water exists in a single phase only. Milk that you buy in the store has a uniform composition throughout and does not separate upon standing so it is a homogeneous mixture.
Salt water acts as if it were a single substance even though it contains two substances. A homogeneous mixture however can only be separated using chemical means. Salt water is a homogeneous mixture or a solution.
All solutions would be considered homogeneous because the dissolved material is present in the same amount throughout the solutionA heterogeneous mixture is a mixture in which the composition is not uniform throughout the mixture. Get an answer for Is salt a heterogeneous mixture a compound or a homogenous mixture and find homework help for other Science questions at eNotes. If you want to separate the salt and the water you must evaporate the water away in order to be left with water-free salt.
Another word for a homogeneous mixture is solutionThus a combination of salt and steel wool is a heterogeneous mixture because it is easy to see which particles of the matter are salt crystals and which are steel wool. The concentration varies from place to place and elements to elements in the mixture. We can separate components of a mixture by simple physical methods.
Iodisation is only the physical process of mixing the iodine compound with common saltIodised salt so obtained is a mixture a heterogeneous mixture. Saltwater is a basic mixture of salt NaCl and water H2O. A heterogeneous mixture is a type of mixture that allows the components to be seen as two or more phases are present.
Therefore salt water is a homogeneous mixture. The difference is that the composition of the substance is always the same. Chocolate chip cookies are a heterogeneous mixture.
Soil is composed of small pieces of a variety of materials so it is a heterogeneous mixture. But it is also called a heterogeneous mixture because of the presence of impurities and insoluble components like sands shells made up of calcium carbonate and microbes in it. Saltwater contains dissolved salt so its a homogeneous mixture as we cant separate salt from it directly.
For example we can separate common salt dissolved in water by the process of evaporation. Salt is soluble in water which would give a homogeneous mixture. The amount of salt in the salt water can vary from one sample to another.
Salt water is made by mixing salt NaCl in water. Often it is easy to confuse a homogeneous mixture with a pure substance because they are both uniform. In contrast examples of homogeneous mixtures include air salt water and steel.
Is tea a heterogeneous mixture. Class 9 Science Free PDF Download that will help you in. Salt is a white.
In a homogeneous mixture the concentration is the same at every place. The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample. A mixture of sand and common salt is regarded as a heterogeneous mixture.
So this depends on the concentration of the mixture.

Matter Pure Substance Elementcompound Mixture Homogeneous Solution Colloid Heterogeneous Suspension Solutions Cell Processes Forms Of Matter

Example Of Elements Compounds And Mixtures Compounds And Mixtures Elements Compounds And Mixtures Mixtures

General Chemistry Online Faq Solutions Why Does Salt Melt Ice Chemistry Education Chemistry Teaching Chemistry

Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Middle School Science Resources

Is Matter Around Us Pure School Help By Gunjan Pure Products School Help Homogeneous Mixture

Mixtures Homogeneous Heterogeneous And Solutions Power Point Types Of Mixtures Heterogeneous Mixture Chemical And Physical Changes

Classification Of Matter Ultimate Card Sort For Pure Substances And Mixtures Sorting Cards Creative Teaching Teacher Preparation

Get Examples Of Pure Substances Pure Products Substances Middle School Science Classroom
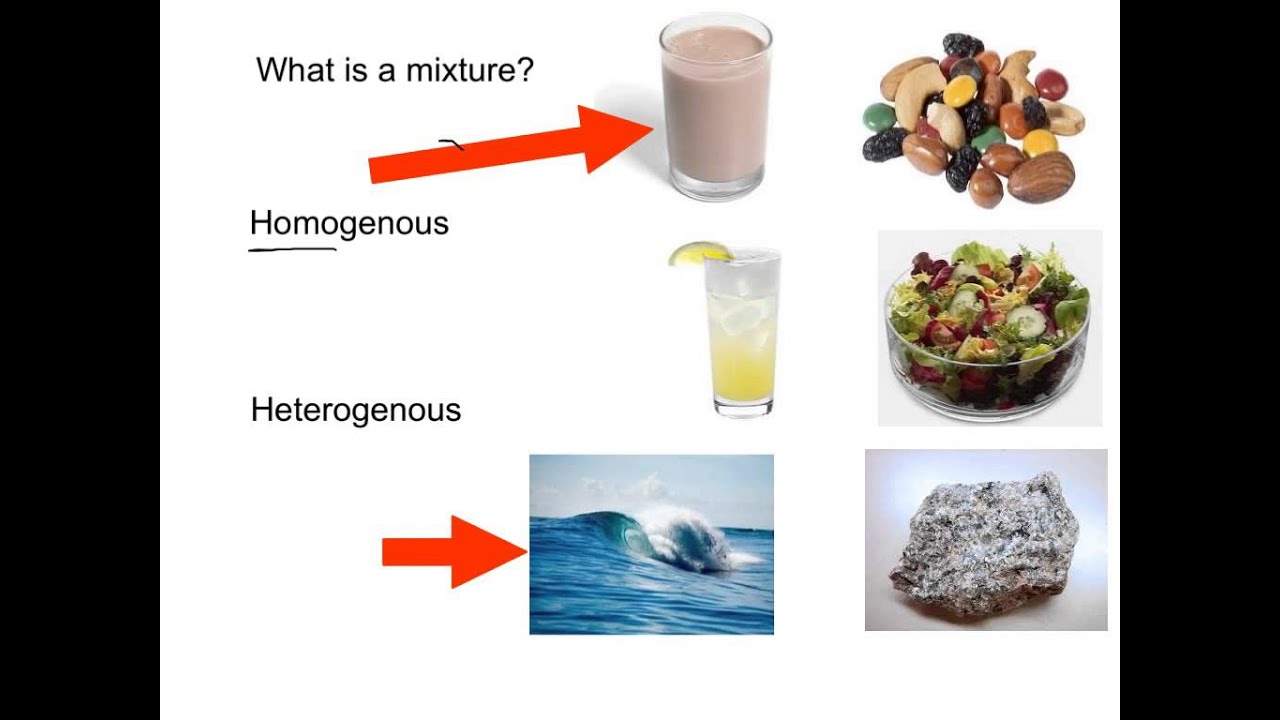
Pin On Middle School Chemistry

Pure Substances And Mixtures Pure Products Elements Compounds And Mixtures Compounds And Mixtures

Selina Concise Chemistry Class 6 Icse Solutions Chapter 5 Pure Substances And Mixtures Separation Of Mixtures Ncert Chemistry Class Chemistry Science Notes

Let S Mention The Difference Between A Solution And A Heterogeneous Mixture A Solution Is A Comb Chemical Science Solutions And Mixtures Heterogeneous Mixture

Esquema Puro De Sustancias Y Mezclas Quimica Educativa Para Los Ninos Ilustracion De Vector Pure Products Elements Compounds And Mixtures Chemistry For Kids

Mixtures And Pure Substances Heterogeneous Mixture Pure Products Mixtures

Mixtures And Solutions 5th Grade Science Middle School Science Experiments Physical Science Lessons

Examples Of Pure Substances Pure Products How To Make Tea Types Of Mixtures

Pin By Carrie Flanagan On School Stuff Chemical Science Matter Science Chemistry Classroom

More Science At Home Separating Salt Pepper Using A Balloon Stuffed Peppers Heterogeneous Mixture Sand And Water

0 Response to "is salt a heterogeneous mixture"
Post a Comment